Law of Chemical Equilibrium and Equilibrium Constants
Law of Chemical Equilibrium and Equilibrium Constants: Overview
This topic covers concepts, such as, Law of Mass Action, Forward Rate Constant, Backward Rate Constant, Equilibrium Constant, Active Mass, Equilibrium Law or Law of Chemical Equilibrium & Equilibrium Equation etc.
Important Questions on Law of Chemical Equilibrium and Equilibrium Constants
An expression for the backward rate constant of the reaction, is
The reaction is elementary order reaction opposed by elementary secondary order reaction. When started with equimolar amounts of and at equilibrium, it is found that the concentration of is twice that of Specific rate constant for forward reaction is The specific rate constant for backward reaction is
For the reaction,
What is when the equilibrium concentration of and ?
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
Calculate for the reaction,
Given
Given
Which relation is correct?
Find the number of reaction which will be favoured in backward direction:
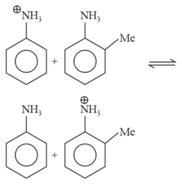
Active mass of mole pure water:
In which case does the reaction goes farthest to completion :
The mass ratio of steam and gas at equilibrium is . The value of equilibrium constant is :
Consider the equilibrium .The equilibrium constant is given by (when
At constant temperature and atm pressure, dissociate then at what pressure dissociate :-
(Use )
For gaseous reactions values of equilibrium constants are given:
The correct relation is:-
The equilibrium constant expression for a gaseous reaction is:-
The correct reaction would be :-
Initial concentration of is . What will be the equilibrium concentration of ?
Select the correct graph for rate versus time. rate of forward reaction and rate of backward reaction)
Consider the following equilibrium :
Find the approximate value of for the given equilibrium reaction, when moles of and mole of are allowed to react in a container of capacity and the following equilibrium is established:
, given that the equilibrium mixture requires of acidified solution.
At the for the following reaction is
At equilibrium, it was observed that:
Find the value of at equilibrium.
A one-litre vessel containing and are heated at , where the total pressure was found to be . Assuming nitrogen to be inert, determine for the following decomposition reaction:
(Put your answer by multiplying with and round off to the nearest integer)
What will be the value of active mass taken for solids?
